Knock in mice
Knock in mice
What are knock in mice?
Knock in mice have an insertion in a specific locus in order to produce genetically modified mice for customised research purposes. Reporter genes, human genes, related genes from the same organism, or point mutations can be introduced into the protein-coding region of a gene to produce knock-in mice. The difference between the knock in and knockout approach is that knock-in mouse models are utilised to drive expression of an exogenous transgene or gene mutation, whereas knockout mice aim to disrupt the expression of a specific wild-type gene.
What are knock in mice used for?
Researchers use knock in mouse models to mimic human genetic disorders, understand embryonic development and evaluate therapeutics by assigning gene functions, dissecting genetic pathways and manipulating the cellular or biochemical properties of proteins. Read our research features and publications for examples of knock in research.
Types of knock in mouse designs:
- Reporter – Track gene expression or the lineage specification and differentiation of desired cell types.
- Point mutation – Study functional gain/loss of a protein with a subtle mutation by altering one or more nucleotides.
- Humanization – Study antibody or small molecule drugs in mice expressing the human drug target.
- Targeted transgenics at a ‘safe harbor’ locus – Study novel transgenes in the absence of random insertion effects.

How to make knock in mice?
Knock in mice are generated by gene targeting via homologous recombination of a targeting construct containing the desired knock in sequence at the target genomic locus. Knock in mouse models range from the introduction of a single point mutation within a gene to the replacement of an entire gene within its human orthologue or other large DNA sequence and can be generated via conditional or non-conditional approaches. Germline transmission has been achieved for all projects to date with leading timelines, thanks to our proprietary goGermline™ technology.
Non-conditional KI models
Non-conditional (constitutive) knock-in mutations are generated by knocking in a foreign sequence into a targeted locus which expresses the inserted sequence throughout the development and life of the model with no options or conditionality. Some of the common designs for non-conditional models are reporter-tagged mice and recombinase mice. However, we recommend the conditional approach whenever possible; the non-conditional approach is not recommended for transgene knock-ins which could affect development, as it can lead to lethality, sterility and developmental defects in ES cells, chimeras, and heterozygous mutant mice.
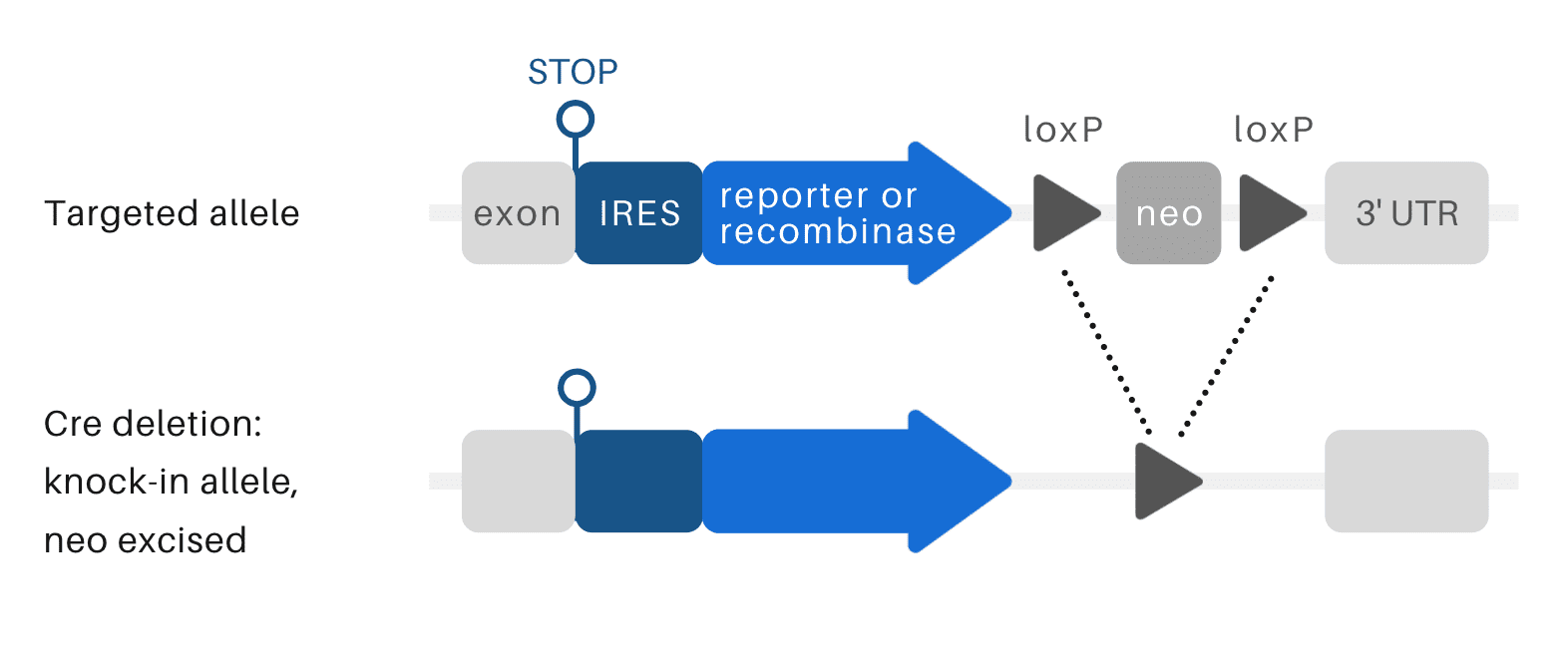
Reporter/recombinase KI mice
Reporter knock-in mouse models can be used to track the expression of a targeted gene. Commonly used fluorescence reporters are green fluorescent protein (GFP) and red fluorescent protein (RFP).
Recombinase knock-in mouse models carry recombinases such as Cre. These mouse models are developed to cross with gene-targeted mice carrying the conditional knock-in or conditional knockout alleles.
Conditional KI models
Conditional knock-in mutations allow inducible and tissue-specific transgene expression in combination with Cre/Lox technology, which provides control over gene expression. This approach produces a stable gain of function as it has the ability to spatially and temporally inactivate or activate gene expression. We would typically choose to create our knock-in models (such as a point mutation, humanization or targeted transgenic model) using a conditional approach.
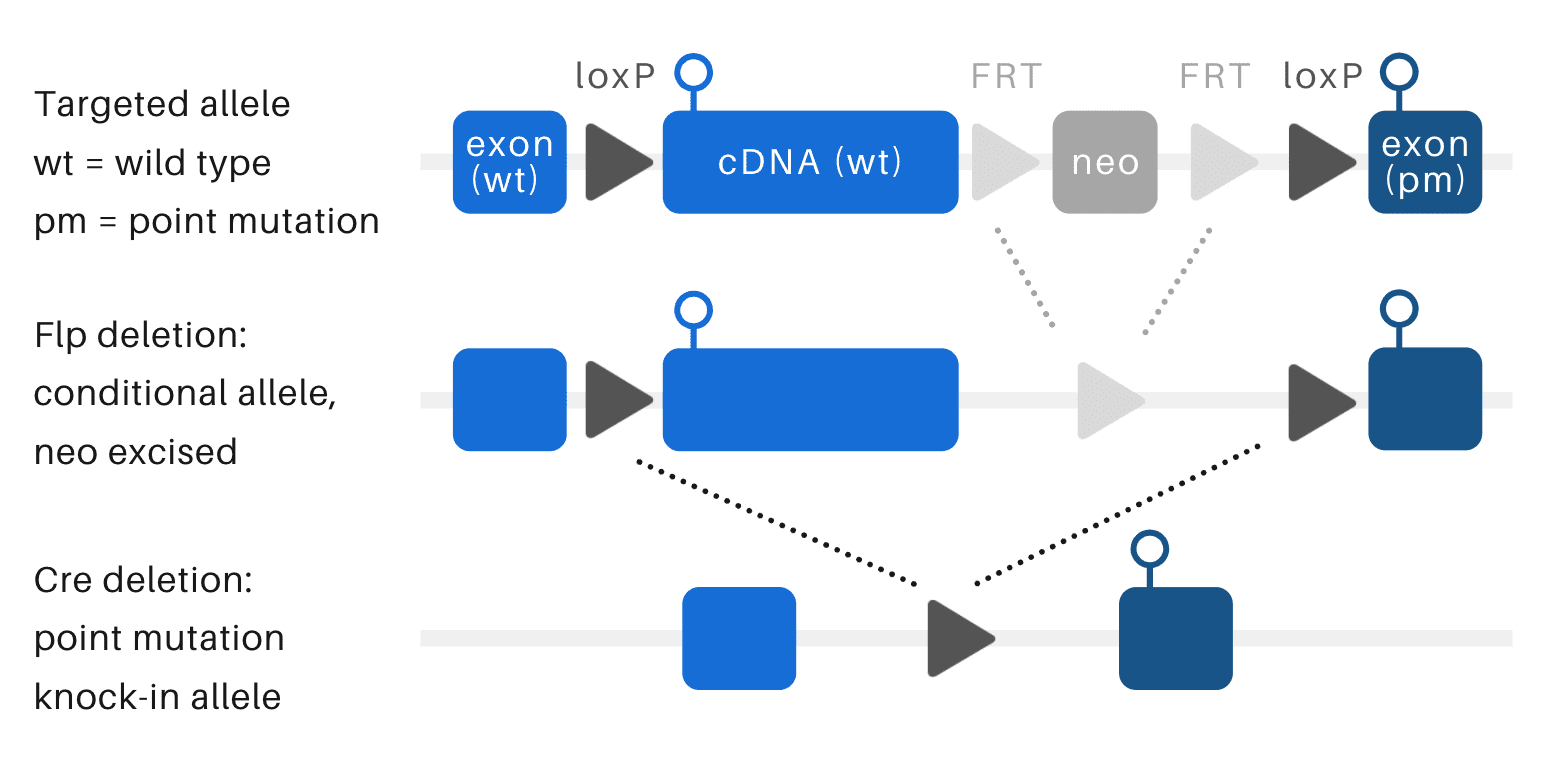
Point mutation/minigene KI mice
This conditional knock-in model uses the Cre/Lox recombination system to express the modified knock-in allele following breeding to a Cre-expressing line. Conditionality is conferred by the presence of a floxed minigene cassette. In the conditional allele, the wild-type mouse protein is expressed from the minigene. Following Cre-mediated recombination, the minigene is excised, permitting expression of the modified knock-in protein.
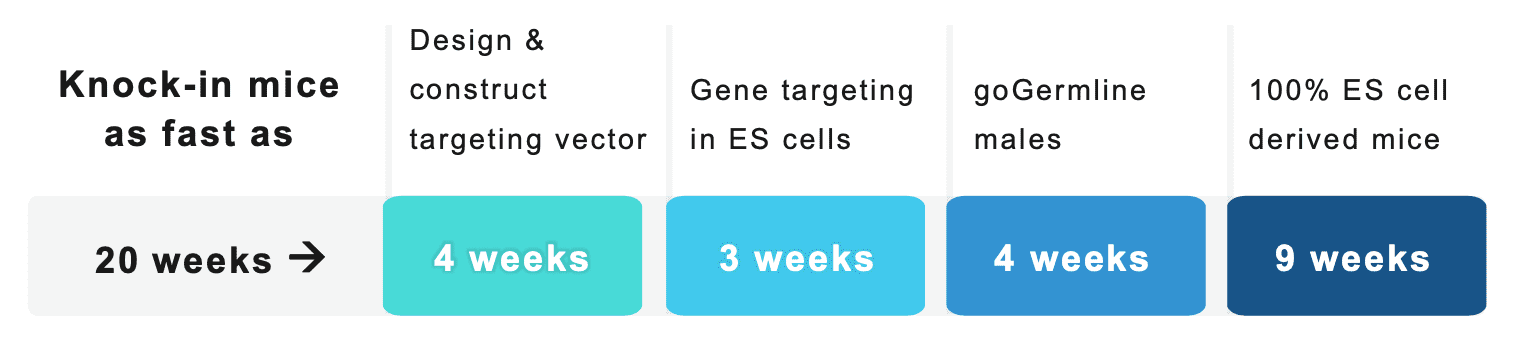
How long does it take to generate knock-in mice?
“No project queues – get started today!”
Ozgene’s Lean management style coupled with patented goGermline technology allows us to generate mouse models in the fastest possible time together with increased certainty of germline transmission.
OzBIG – Genomic replacements up to 240 kb
Ozgene also offers a service called OzBIG for robust creation of large, gene-targeted replacement models. This system enables routine generation of humanized mice with very large (20 to 240 kb) genomic replacements and insertions. OzBIG can also be used to generate knock in mice with large non-humanized, transgenics like complex reporters and synthetic expression systems.
Read more about Ozgene’s Knock in mice
DNL343 is an investigational CNS penetrant eukaryotic initiation factor 2B activator that prevents and reverses the effects of neurodegeneration caused by the integrated stress response
Dynamic allostery drives autocrine and paracrine TGF-β signaling
Unraveling the role of MiR-181 in skin fibrosis pathogenesis by targeting NUDT21
Get in touch
We offer personalised services for your research needs. Request a free quote today.
Please fill out the form and we will respond to your query within two business days. Alternatively, visit our contact page for more ways that you can get in touch with us.
‘I was impressed with the discussions with the Ozgene technical team, prior to us making a decision. Once the project started, regular updates made me feel like I knew what was going on at every step in the process.’
– Prof. Deborah Henderson, Newcastle University, UK

